The Advanced Optical Microscopy Facility in Toronto, Canada, serves University Health Network researchers as well as external academic and industrial users. With over 40 instruments at 4 sites, the AOMF is equipped for nearly any optical microscopy experiment including: confocal, multiphoton, super-resolution, FRAP, FRET, live-cell timelapse, whole-slide scanning, image analysis and more.
Founded in 2002
years of experience
Trusted by over
users a year
With over
instruments
Let us help you produce meaningful data from your images!
Let us help you produce meaningful data from your images!
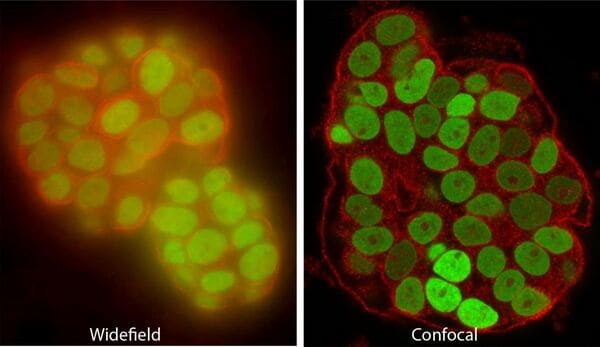
Confocal and multiphoton (two-photon) microscopes use lasers to excite fluorescence, and exclude out-of-focus light to image slices or “optical sections” of the specimen that can be thinner than 1um. Confocals are the AOMF's most popular instruments. Most are equipped for live cells, and some have special capabilities such as super-resolution, fast resonant scanning, or extra-sensitive GaAsP/HyD detectors.
| Model |
Leica Stellaris 5 Confocal microscope
Leica DMi8 CS Premium inverted stand |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405, CFP)
White Light Laser (WLL), 485-790nm in 1nm steps (Alexa 488, GFP, YFP, Alexa 568, Alexa 594, Alexa 647, and Alexa 750/Cy7 and equivalent) |
| Objective lenses |
HC PL FLUOTAR 5x/0.15 NA
HC PL APO 10x/0.40 NA CS2 HC PL APO 20x/0.75 NA CS2 HC PL APO 20x/0.75 NA IMM CORR CS2 (water, glycerol, oil immersion) HCX PL APO 40x/0.95 NA CORR (0.11 - 0.23mm coverslip correction) HC PL APO 63x/1.40 NA Oil immersion CS2 |
| Detectors |
5 HyDs high-sensitivity detectors
Transmitted-light PMT |
| Filters | Fully spectral, 400 to 850nm |
| Incubation | Full CO2 incubator (including temperature, humidity) |
| Advantages and techniques |
High-resolution confocal
NIR dyes (Cy7, Alexa 750) Spectral unmixing Tiling / Stage Overview Live-cell imaging with AFC hardware autofocus Tau-Sense (simple Fluorescence Lifetime Imaging) Galvo scanner (high quality) AND Resonant Scanner (fast - 30fps) |
| Software |
Leica LAS-X software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model |
Leica Stellaris 5 Confocal microscope
Leica DMi8 CS Premium inverted stand |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405, CFP)
448nm (CFP) 488nm (Alexa488, Cy2, FITC, GFP) 514nm (YFP) 561nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) 638nm (Alexa 647, Cy5) |
| Objective lenses |
HC PL FLUOTAR 5x/0.15 NA
HC PL APO 10x/0.40 NA CS2 HC PL APO 20x/0.75 NA CS2 HC PL APO 40x/1.25 NA GLYC motCORR CS2 HC PL APO 63x/1.40 NA Oil immersion CS2 |
| Detectors |
5 HyDs high-sensitivity detectors
Transmitted-light PMT |
| Filters | Fully spectral, 400 to 850nm |
| Incubation | Full CO2 incubator (including temperature, humidity) |
| Advantages and techniques |
High-resolution confocal
Spectral unmixing Tiling / Stage Overview Live-cell imaging with AFC hardware autofocus Galvo scanner (high quality) AND Resonant Scanner (fast - 30fps) |
| Software |
Leica LAS-X software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model |
Leica Stellaris 5 Confocal microscope
Leica DMi8 CS Premium inverted stand |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405, CFP)
White Light Laser (WLL), 485-790nm in 1nm steps (Alexa 488, GFP, YFP, Alexa 568, Alexa 594, Alexa 647, and Alexa 750/Cy7 and equivalent) |
| Objective lenses |
HC PL FLUOTAR 5x/0.15 NA
HC PL APO 10x/0.40 NA CS2 HC PL APO 20x/0.75 NA CS2 HC PL APO 20x/0.75 NA IMM CORR CS2 (water, glycerol, oil immersion) HCX PL APO 40x/0.95 NA CORR (0.11 - 0.23mm coverslip correction) HC PL APO 63x/1.40 NA Oil immersion CS2 |
| Detectors |
5 HyDs high-sensitivity detectors
Transmitted-light PMT |
| Filters | Fully spectral, 400 to 850nm |
| Incubation | Full CO2 incubator (including temperature, humidity) |
| Advantages and techniques |
High-resolution confocal
NIR dyes (Cy7, Alexa 750) Spectral unmixing Tiling / Stage Overview Live-cell imaging with AFC hardware autofocus Tau-Sense (simple Fluorescence Lifetime Imaging) Galvo scanner (high quality) AND Resonant Scanner (fast - 30fps) |
| Software |
Leica LAS-X software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model |
Leica Stellaris 5 Confocal microscope
Leica DMi8 CS Premium inverted stand |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405, CFP)
448nm (CFP) 488nm (Alexa488, Cy2, FITC, GFP) 514nm (YFP) 561nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) 638nm (Alexa 647, Cy5) |
| Objective lenses |
HC PL FLUOTAR 5x/0.15 NA
HC PL APO 10x/0.40 NA CS2 HC PL APO 20x/0.75 NA CS2 HC PL APO 40x/1.25 NA GLYC motCORR CS2 HC PL APO 63x/1.40 NA Oil immersion CS2 |
| Detectors |
5 HyDs high-sensitivity detectors
Transmitted-light PMT |
| Filters | Fully spectral, 400 to 850nm |
| Incubation | Full CO2 incubator (including temperature, humidity) |
| Advantages and techniques |
High-resolution confocal
Spectral unmixing Tiling / Stage Overview Live-cell imaging with AFC hardware autofocus Galvo scanner (high quality) AND Resonant Scanner (fast - 30fps) |
| Software |
Leica LAS-X software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model |
Zeiss LSM700 Confocal microscope
Zeiss AxioObserver 200M inverted stand |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405)
488nm (Alexa488, Cy2, FITC, GFP) 555nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) 639nm (Alexa 647, Cy5) |
| Objective lenses |
FLUAR 5x/0.25 NA
FLUAR 10x/0.50 NA Plan-Apochromat 20x/0.8 NA Plan-Apochromat 40x/1.4 NA oil immersion Plan-Apochromat 63x/1.4 NA oil immersion C-APO 63x/1.2 NA water immersion |
| Detectors |
2 standard sensitivity PMT detectors (multiplexing for up
to 4 channels sequentially)
Transmitted-light PMT |
| Filters | Fully spectral, 400 to 700nm |
| Incubation | None |
| Advantages and techniques |
High-resolution confocal
Spectral unmixing |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model |
Zeiss LSM710 Confocal and multiphoton (two-photon)
microscope
Zeiss AxioExaminer upright stand |
| Lasers, fluorophores |
Coherent Chameleon Discovery Laser
488nm (Alexa488, Cy2, FITC, GFP) 561nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) 633nm (Alexa 647, Cy5) |
| Objective lenses |
FLUAR 5x/0.25 NA
FLUAR 10x/0.50 NA W Plan-Apochromat 20x/1.0 NA water immersion Plan-Apochromat 63x/1.4 NA oil immersion |
| Detectors |
3 standard PMT detectors
4-channel non-descanned (NDD) detector for deep imaging |
| Filters | Fully spectral, 400 to 700nm |
| Incubation | None |
| Advantages and techniques |
In vivo imaging
High-resolution confocal Multiphoton imaging for deep tissue penetration Spectral unmixing Second- and Third-harmonic generation imaging |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model |
Leica TCS SP8 Confocal microscope
Leica DMi8 CS inverted stand |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405)
488nm (Alexa488, Cy2, FITC, GFP) 552nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) 638nm (Alexa 647, Cy5) |
| Objective lenses |
HC PL FLUOTAR 5x/0.15 NA
HC PL APO 10x/0.40 NA CS2 HC PL APO 20x/0.75 NA CS2 HCX PL APO 40x/0.85 NA CS, Corr 0.11 - 0.23mm HC PL APO 63x/1.40 NA Oil immersion CS2 |
| Detectors |
3 standard sensitivity PMT detectors + 2 HyD
high-sensitivity detectors
Transmitted-light PMT |
| Filters | Fully spectral, 400 to 700nm |
| Incubation | None |
| Advantages and techniques |
High-resolution confocal
Spectral unmixing Tiling / Stage Overview AFC hardware autofocus |
| Software |
Leica LAS-X software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model |
Leica TCS SP8 STED3X Confocal and Super-resolution
microscope
Leica DMi8 CS inverted stand FALCON FLIM/FCS TauSTED |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405)
440nm (CFP) White Light Laser (WLL), 475-675nm in 1nm steps (Alexa 488 to Cy5 and everything in between) |
| Objective lenses |
HC PL APO 10x/0.40 NA CS2
HC PL APO 20x/0.75 NA CS2 HC PL APO 63x/1.40 NA Oil immersion CS2 HC PL APO 63x/1.20 NA Water immersion CS2 motCorr (by request) HC PL APO 93x/1.30 NA Glycerol immersion CS2 motCorr HC PL APO 100x/1.40 NA Oil immersion CS2 |
| Detectors |
3 standard sensitivity PMT detectors + 2 HyD
high-sensitivity detectors
Transmitted-light PMT |
| Filters | Fully spectral, 400 to 700nm |
| Incubation | Full CO2 incubator (including temperature, humidity) |
| Advantages and techniques |
High-resolution confocal of fixed and live cells
STED super-resolution with 592nm, 660nm, and 775nm depletion lasers TauSTED super-resolution (STED with FLIM for gentler STED) Fluorescence Lifetime Imaging Microscopy (FLIM) Fluorescence Correlation Spectroscopy (FCS) Hyvolution 2 deconvolution Spectral unmixing Tiling / Stage Overview AFC hardware autofocus |
| Software |
Leica LAS-X software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model |
Nikon A1R Confocal microscope with resonant scanner
Nikon Eclipse Ti inverted stand |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405)
445nm (CFP) 488nm (Alexa488, Cy2, FITC, GFP) 514 nm (YFP) 561nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) 640nm (Alexa 647, Cy5) |
| Objective lenses |
Plan Apo 10x/0.45 NA
Plan Apo 20x/0.75 NA Plan FL 40x/1.3 NA oil Plan Apo, nano-crystal, 60x/1.4 NA, oil immersion Plan Apo, IR, 60x/1.7 NA, water immersion NIR Apo 40x/0.8 NA, water immersion |
| Detectors |
2 standard PMT detectors + 2 GaAsP high-sensitivity
detectors
Transmitted-light PMT |
| Filters | Fully spectral, 400 to 750nm |
| Incubation | OKO Lab stage-top incubator |
| Advantages and techniques |
High-Speed resonant scanning confocal for imaging
dynamics; and standard galvanometer scanning mirrors for
high-quality imaging of fixed samples
MCL nano-drive allows fast Z-stack imaging Live cell imaging Perfect focus hardware autofocus system |
| Software |
Nikon Element software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model |
Yokogawa CSU-10 Spinning-disk confocal
Olympus IX81 inverted stand |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405)
491nm (Alexa488, Cy2, FITC, GFP) 561nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) 640nm (Alexa 647, Cy5) 730 nm (Alexa 700, Alexa 750, Cy7) |
| Objective lenses |
UPlan FL 10x/0.30 NA
UPlan SApo 20x/0.75NA UPlan FL 40x/0.75 NA UPlan Apo 60x/1.2 NA water immersion Plan Apo 63x/1.4 NA oil immersion |
| Camera | Hamamatsu EM-CCD C9100-13 |
| Incubation | LCI Stage incubator |
| Advantages and techniques |
Piezo Z-axis stage allows fast z-stack imaging
Live cell imaging |
| Software |
Volocity software
FIJI ImageJ (free download - 2D analysis) |
| Model |
Yokogawa CSU-10 Spinning-disk confocal
Olympus IX81 inverted stand |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405)
491nm (Alexa488, Cy2, FITC, GFP) 561nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) 640nm (Alexa 647, Cy5) |
| Objective lenses |
UPlan FL 10x/0.30 NA
UPlan SApo 20x/0.75NA UPlan FL 40x/0.75 NA UPlan Apo 60x/1.2 NA water immersion Plan Apo 63x/1.4 NA oil immersion |
| Camera | Hamamatsu EM-CCD C9100-13 |
| Incubation | LCI Stage incubator |
| Advantages and techniques |
Piezo Z-axis stage allows fast z-stack imaging
Live cell imaging |
| Software |
Volocity software
FIJI ImageJ (free download - 2D analysis) |
| Model |
Zeiss LSM880 Confocal microscope with AiryScan
Zeiss AxioObserver Stand |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405)
488nm (Alexa488, Cy2, FITC, GFP) 555nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) 639nm (Alexa 647, Cy5) |
| Objective lenses |
Plan-Apochromat 10x/0.45 NA
Plan-Apochromat 20x/0.80 NA Plan-Apochromat 40x/0.95 NA Plan-Apochromat 63x/1.4 NA oil immersion |
| Detectors |
2 standard sensitivity PMT detectors
1 high-sensitivity GaAsP detector |
| Filters | Fully spectral, 400 to 700nm |
| Incubation | Cell culture and 24-well plate format |
| Advantages and techniques |
High-resolution and extended resolution (AiryScan)
confocal
Spectral unmixing Live cell confocal imaging - FRET, FRAP |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model |
Leica TCS SP8 MP Confocal and multiphoton microscope
Leica DM8 physiological upright stand |
| Lasers, fluorophores |
Coherent Chameleon Discovery Laser
555nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) |
| Objective lenses | HC-IRAPO 25X / 1.0 NA water dipping lens |
| Detectors |
2 Internal detectors
|
| Filters | Fully spectral, 400 to 700nm |
| Incubation | Thermal plate |
| Advantages and techniques |
In vivo imaging
High-resolution confocal High-speed resonant confocal Multiphoton imaging for deep tissue penetration Spectral unmixing Second- and Third-harmonic generation imaging |
| Software |
Leica LAS-X (Free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
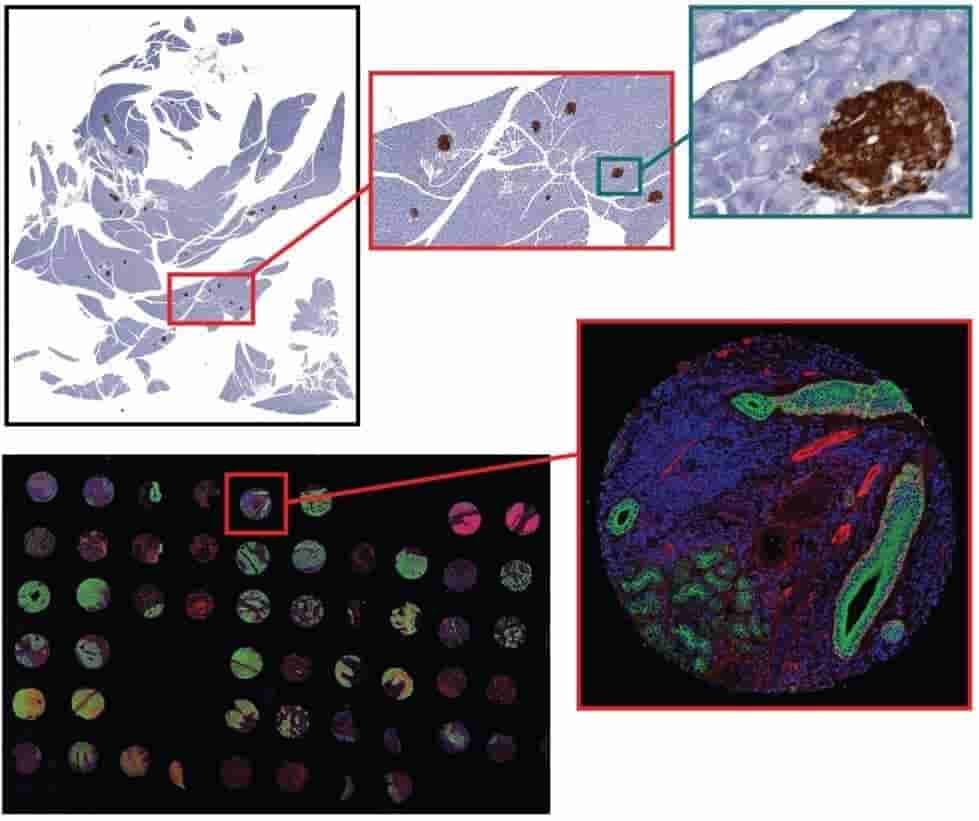
For tissue sections from 4um to 12um thick, AOMF staff will digitize your entire slide at high resolution starting at just 8.75/slide for brightfield and $15/slide for fluorescence (see Fees). The resulting images range from 100MB to 10GB per slide, and therefore special (free) software is required for viewing them. Applications include virtual microscopy (pan, zoom, and “snap” a picture of any field of view) and whole-slide analysis (proprietary HALO software required).
| Model |
Aperio AT2 brightfield scanner (Leica Biosystems
400 slide capacity |
| Objective lens |
UPlanS Apo 20x / 0.75 NA (high-resolution 20x objective)
2x doubler for 40x |
| Pixel Size / Resolution |
20x: 0.5µm/pixel
40x: 0.25µm/pixel |
| Input slide sizes |
Standard slide: 1” x 3”
Whole-mount slide: 2” x 3” |
| Turn-around time | Typically 2 to 5 business days |
| Software |
Aperio Imagescope software (free download)
Halo by IndicaLabs (offline, licensed) |
| Model |
Zeiss AxioScan
100 slide capacity |
| Filters / fluorophores |
Chroma ET DAPI 49000 (DAPI)
Chroma ET EGFP 49002 (Alexa488, Cy2, FITC, GFP) Chroma ET CY3/TRITC 49004 (Alexa555, Alexa568, Cy3, DsRed, RFP) Chroma ET mCherry TexasRed 49008 (Alexa594, TexasRed, mCherry) Chroma ET Cy5 49006 (Alexa 647, Cy5) * Note users must choose either Cy3 or TxRed probe, not both |
| Objective lens |
Fluar 2.5x / 0.12 NA
Plan Apochromat 5x / 0.16 NA Plan Apochromat 10x / 0.45 NA Plan Apochromat 20x / 0.80 NA Plan Apochromat 40x / 0.95 NA |
| Pixel Size / Resolution |
Standard resolution: 20x (0.25µm/pixel)
High resolution: 40x: (0.12µm/pixel) |
| Input slide sizes |
Standard slide: 11” x 31”
Whole-mount slide: 21” x 31” |
| Turn-around time | Typically 5 to 10 business days |
| Software |
Zeiss Zen software (free download)
Halo by Indica Labs (offline, licensed) |
| Licenses |
5-seat license for HALO
1 demo license for HALO-AI Deep Learning (expires Jan 2021) |
| Locations |
5 local licenses are installed on high-end desktop
computers
|
| Modules / Add-ons |
Tissue Classifier
Tissue Microarray (TMA) Serial Section Analysis Batch analysis (typically 50 slides can be analyzed overnight) Cytonuclear IHC / Cytonuclear FL Area Quantification / Area Quantification FL Object Colocalization / Object Colocalization FL Vacuole Islet IHC / Islet FL Highplex FL / Multiplex IHC ISH (RNAScope) / FISH (RNAScope) |
| File Formats |
Aperio (SVS, AFI)
Zeiss (CZI) 3D Histech (MRXS) Hamamatsu (NDPI, NDPIS) Nikon (ND2) Olympus (VSI) Leica (SCN, LIF) Ventana (BIF) Perkin Elmer (QPTIFF, component TIFF) Non-proprietary (JPG, TIF) |
| Typical workflow |
|
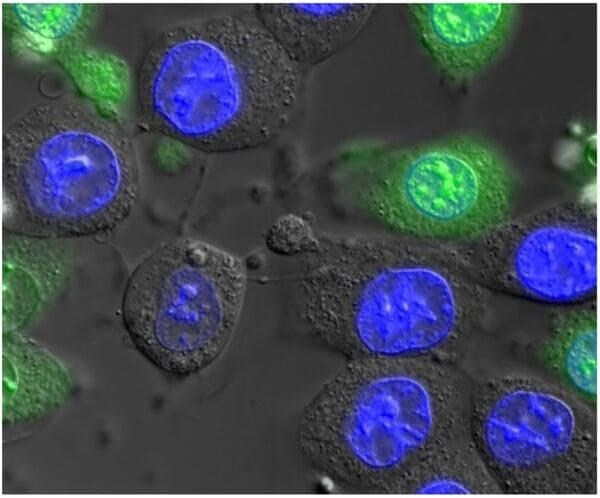
For applications that don't demand confocal microscopy, our widefield fluorescence microscopes incorporate motorization and high-end components beyond what you would find in a typical lab. Most are equipped with fast, high-resolution cameras, and some have full incubator boxes for long-term timelapse imaging, or macroscope lenses for large fields of view. Available contrasts include fluorescence, brightfield, phase contrast, Differential Interference Contrast (DIC), polarized light, and darkfield.
| Model | Zeiss AxioObserver.Z1 inverted stand |
| Light source | X-Cite Xylis II LED |
| Filter sets |
|
| Objective lenses |
Fluar 5x/0.25 NA
Plan-Apochromat 10x/0.45 NA Phase Contrast Plan-Apochromat 20x/0.80 NA Plan-Apochromat 40x/0.95 NA LD A-Plan 32X/0.40 NA Long working distance (plastic) phase contrast Plan-Apochromat 63x/1.4 NA oil immersion - available upon request |
| Camera | Hamamatsu ORCA-Flash 4.0 (4 MP sCMOS camera) |
| Incubation | Full PECON incubation chamber with temperature, humidity, and CO2. Inserts for Ibidi chambered coverslips, 35 mm dishes, and multiwell plates with low-elevation coverslip bottom. |
| Advantages and techniques |
Fixed slides (widefield immunofluorescence)
Live cell imaging, both fluorescence and phase contrast/DIC High-speed widefield imaging Tiling/stitching Fluorescence, phase contrast, DIC |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) |
| Model | Zeiss AxioObserver.Z1 inverted stand |
| Light source | X-Cite Xylis II LED |
| Filter sets |
|
| Objective lenses |
Plan-Apochromat 5x/0.15 NA PH1
Fluar 10x/0.5 NA Plan-Apochromat 10x/0.25 NA PH1 Plan-Apochromat 20x/0.80 NA Plan-Apochromat 63x/1.4 NA oil immersion |
| Camera | Hamamatsu ORCA-Flash 4.0 (4 MP sCMOS camera) |
| Incubation | Full PECON incubation chamber with O2 module |
| Advantages and techniques |
Live cell imaging
Multi-location timelapse imaging (up to 48 hours) Definite focus hardware autofocus system Widefield immunofluorescence |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) |
| Model | Zeiss AxioImager.Z1 upright stand |
| Light source | X-Cite eXacte metal halide fluorescence lamp |
| Filter sets |
Narrow cubes optimized for FISH
|
| Objective lenses |
EC Plan-Neofluar 10x/0.3
Plan Neofluar 16x/0.5 NA multi-immersion Plan-Neofluar 20x/0.5 NA Plan-Neofluar 40x/0.75 NA Plan-Apochromat 63x/1.4 NA oil immersion Plan-Neofluar 100x/1.3 NA oil immersion |
| Cameras |
Hamamatsu ORCA-Flash 4.0 (4 MP sCMOS camera)
CRI Nuance spectral camera (available upon request) |
| Advantages and techniques |
Fixed slides (widefield immunofluorescence)
Fluorescence In Situ Hybridization (FISH) |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) |
| Model | Zeiss AxioZoom.V16 upright macroscope |
| Light source | X-Cite eXacte metal halide lamp |
| Filter sets |
|
| Objective lenses | Plan Neofluar Z 1X/0.25 NA |
| Camera | Hamamatsu ORCA-Flash 4.0 (4 MP sCMOS camera) |
| Incubation | None |
| Advantages and techniques |
Large FOV imaging
Widefield fluorescence stitching and tiling Gross specimen imaging |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) |
| Model | Leica MZFLIII upright stereomicroscope |
| Light sources |
X-Cite 120 metal halide lamp (fluorescence)
Intralux 5000 halogen (transmitted / reflected light) |
| Filter sets |
|
| Objective lenses |
PlanApo 0.8 to 10x / 0.12 NA
Macro lens |
| Camera | Lumenera Infinity 3-6URFC colour camera (6MP) |
| Advantages and techniques |
Dissecting
Large FOV imaging Gross specimen imaging |
| Software |
Micromanager software
FIJI ImageJ (free download - 2D analysis) |
| Model | Zeiss inverted stand |
| Light source | X-Cite 120 LED Boost |
| Filter sets |
|
| Objective lenses |
Plan-Apochromat 10x/0.45 NA
Plan-Apochromat 20x/0.80 NA LD Plan-Neofluar 40x/0.6 Korr Ph 1 Ph 2 Plan-Apochromat 63x/1.4 NA oil immersion Plan-Apochromat 100x/1.4 NA oil immersion |
| Camera | Hamamatsu ORCA-Flash 4.0 (4 MP sCMOS camera) |
| Advantages and techniques |
Fixed slides (widefield immunofluorescence)
motorized Z-stacking |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) |
| Model | Zeiss AxioObserver 7 inverted stand |
| Light source | X-Cite 120 LED Mini |
| Filter sets |
|
| Objective lenses |
Fluar 5x/0.25 NA
Plan-Apochromat 10x/0.45 NA Plan-Apochromat 20x/0.80 NA Plan-Apochromat 40x/0.95 NA LD Achroplan 40X/0.60 NA Long working distance (plastic) phase contrast Plan-Apochromat 63x/1.4 NA oil immersion |
| Camera | Hamamatsu ORCA-Flash 4.0 (4 MP sCMOS camera) |
| Incubation | Cell culture and 24-well plate format |
| Advantages and techniques |
Fixed cells (widefield immunofluorescence)
Live cell imaging High-speed widefield imaging Multi-location timelapse imaging Calcium imaging Tiling/stitching Fluorescence, phase contrast, DIC |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (licensed) Huygens deconvolution (licensed) |
| Model | Zeiss AxioZoom.V16 upright macroscope |
| Light source | X-Cite 120 metal halide lamp |
| Filter sets |
|
| Objective lenses | Plan Neofluar Z 1X/0.25 NA |
| Camera | Hamamatsu ORCA-Flash 4.0 (4 MP sCMOS camera) |
| Incubation | None |
| Advantages and techniques |
Large FOV imaging
Widefield fluorescence stitching and tiling Gross specimen imaging |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) |
A comprehensive investigation of cancer genes and selected non-cancer genes within patient blood and tumour samples can provide information about diagnosis, patient risk stratification and response to therapy, as well as help determine potential application of targeted therapies for different malignancies.
| Model |
Zeiss LSM710 Confocal and multiphoton (two-photon)
microscope
Zeiss AxioExaminer upright stand |
| Lasers, fluorophores |
Coherent Chameleon Discovery Laser
488nm (Alexa488, Cy2, FITC, GFP) 561nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) 633nm (Alexa 647, Cy5) |
| Objective lenses |
FLUAR 5x/0.25 NA
FLUAR 10x/0.50 NA W Plan-Apochromat 20x/1.0 NA water immersion Plan-Apochromat 63x/1.4 NA oil immersion |
| Detectors |
3 standard PMT detectors
4-channel non-descanned (NDD) detector for deep imaging |
| Filters | Fully spectral, 400 to 700nm |
| Incubation | None |
| Advantages and techniques |
In vivo imaging
High-resolution confocal Multiphoton imaging for deep tissue penetration Spectral unmixing Second- and Third-harmonic generation imaging |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model | Leica MZFLIII upright stereomicroscope |
| Light Sources |
X-Cite 120 metal halide lamp (fluorescence)
Intralux 5000 halogen (transmitted / reflected light) |
| Filter sets |
|
| Objective lenses |
PlanApo 0.8 to 10x / 0.12 NA
Macro lens |
| Camera | Lumenera Infinity 3-6URFC colour camera (6MP) |
| Advantages and techniques |
Dissecting
Large FOV imaging Gross specimen imaging |
| Software |
Micromanager software
FIJI ImageJ (free download - 2D analysis) |
| Model | Zeiss AxioZoom.V16 upright macroscope |
| Light Source | X-Cite eXacte metal halide lamp |
| Filter sets |
|
| Objective lenses | Plan Neofluar Z 1X/0.25 NA |
| Camera | Hamamatsu ORCA-Flash 4.0 (4 MP sCMOS camera) |
| Incubation | None |
| Advantages and techniques |
Large FOV imaging
Widefield fluorescence stitching and tiling Gross specimen imaging |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) |
| Model | Xenogen IVIS Spectrum 200 (by Perkin Elmer) |
| Imaging modalities |
Bioluminescence (2D and 3D)
Fluorescence (2D and 3D) Spectral imaging and unmixing |
| Filter sets |
10 excitation filters covering 430nm to 750nm, 30nm
bandwidth
18 emission filters covering 500nm to 850nm, 20nm bandwidth |
| Fields of View |
A: 3.9 cm (small dishes or tissues)
B: 6.5 cm (1 mouse) C: 12.5 cm (3 mice) D: 22.5 cm (5 mice) |
| Camera |
Andor iKon DZ436:
|
| Advantages and techniques |
High-throughput in vivo imaging
Sensitive, non-invasive imaging of tumours |
| Software | Living Image 4.5 |
| Model |
Leica SP8 Confocal microscope
Leica DM8 physiological upright stand |
| Lasers, fluorophores |
Coherent Chameleon Discovery Laser
555nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) |
| Objective lenses | HC-IRAPO 25X / 1.0 NA water dipping lens |
| Detectors |
2 Internal detectors
|
| Filters | Fully spectral, 400 to 700nm |
| Incubation | Thermal plate |
| Advantages and techniques |
In vivo imaging
High-resolution confocal High-speed resonant confocal Multiphoton imaging for deep tissue penetration Spectral unmixing Second- and Third-harmonic generation imaging |
| Software |
Leica LAS-X (Free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (licensed) Huygens deconvolution (licensed) |
The best lateral resolution achievable on a standard confocal fluorescence microscope is about 200nm. The AOMF's super-resolution microscopes can extend this down to 120nm (Airyscan), 50nm (STED), or even 20nm (GSD/STORM). Special sample preparation may be required, so consult with AOMF staff before preparing your samples. For 3D datasets, deconvolution can further improve the resolution.
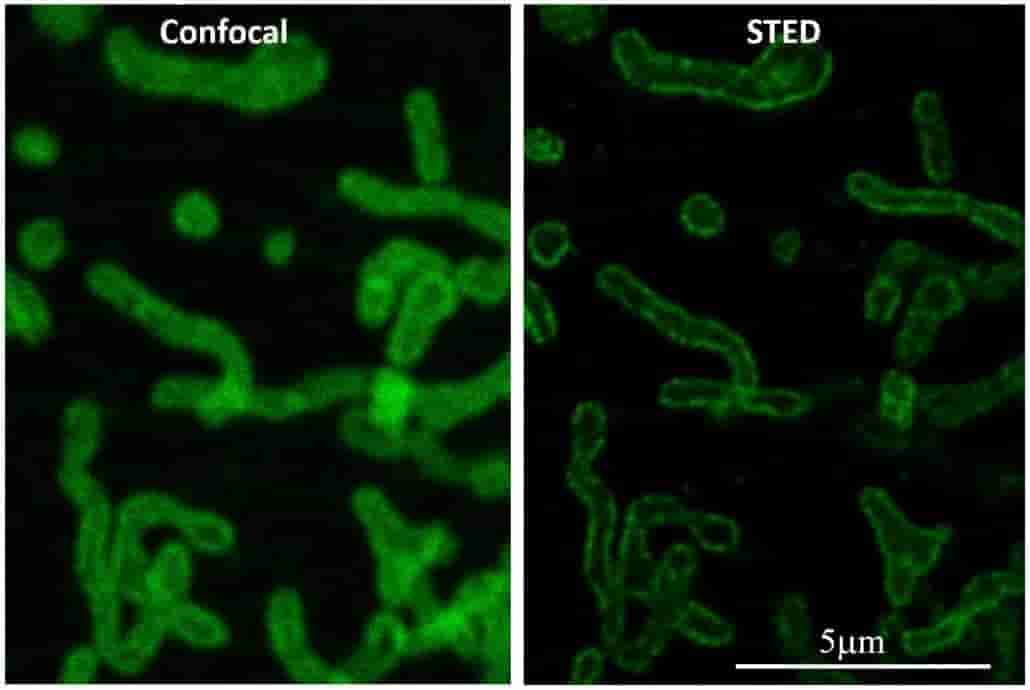
| Model |
Leica TCS SP8 STED3X Confocal and Super-resolution
microscope
Leica DMi8 CS inverted stand FALCON FLIM/FCS TauSTED |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405)
440nm (CFP) White Light Laser (WLL), 475-675nm in 1nm steps (Alexa 488 to Cy5 and everything in between) |
| Objective lenses |
HC PL APO 10x/0.40 NA CS2
HC PL APO 20x/0.75 NA CS2 HC PL APO 63x/1.40 NA Oil immersion CS2 HC PL APO 63x/1.20 NA Water immersion CS2 motCorr (by request) HC PL APO 93x/1.30 NA Glycerol immersion CS2 motCorr HC PL APO 100x/1.40 NA Oil immersion CS2 |
| Detectors |
3 standard sensitivity PMT detectors + 2 HyD
high-sensitivity detectors
Transmitted-light PMT |
| Filters | Fully spectral, 400 to 700nm |
| Incubation | Full CO2 incubator (including temperature, humidity) |
| Advantages and techniques |
High-resolution confocal of fixed and live cells
STED super-resolution with 592nm, 660nm, and 775nm depletion lasers TauSTED super-resolution (STED with FLIM for gentler STED) Fluorescence Lifetime Imaging Microscopy (FLIM) Fluorescence Correlation Spectroscopy (FCS) Hyvolution 2 deconvolution Spectral unmixing Tiling / Stage Overview AFC hardware autofocus |
| Software |
Leica LAS-X software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (offline, licensed) Huygens deconvolution (offline, licensed) |
| Model |
Leica SR GSD 3D
Leica DMi8 inverted Stand |
| Light sources |
Sola Light Engine LED for regular live cell imaging (DAPI,
FITC, CY3, CY5 filters)
405 nm depletion laser for STORM/GSD super-resolution 488 nm laser (STORM/GSD/TIRF) 560nm laser (STORM/GSD/TIRF) 642nm laser (STORM/GSD/TIRF) |
| Objective lenses |
HC PL Apo 10x/0.45 NA
N PL 20x/0.40 NA PH1 HC PL Apo 20x/0.8 NA HC PL Apo 40x/0.85 NA HC PL Apo 100x/1.47 NA oil immersion, TIRF HC PL Apo 160x/1.43 NA, oil immersion, GSD |
| Cameras |
Andor iXon Ultra 897 EMCCD
PCO Edge sCMOS |
| Incubation | Tokai Hit stage-top incubator |
| Advantages and techniques |
STORM / GSD super-resolution:
Live cell imaging |
| Software |
Leica LAS-X software
FIJI ImageJ (free download - 2D analysis) |
| Model |
Zeiss 880 Confocal with AiryScan
Zeiss AxioObserver 7 Stand |
| Lasers, fluorophores |
405nm (DAPI, Hoechst, Alexa405)
488nm (Alexa488, Cy2, FITC, GFP) 555nm (Alexa555, Alexa568, Alexa594, Cy3, DsRed, TxRed, RFP, mCherry) 639nm (Alexa 647, Cy5) |
| Objective lenses |
Plan-Apochromat 10x/0.45 NA
Plan-Apochromat 20x/0.80 NA Plan-Apochromat 40x/0.95 NA Plan-Apochromat 63x/1.4 NA oil immersion |
| Detectors |
2 standard sensitivity PMT detectors
1 high-sensitivity GaAsP detector |
| Filters | Fully spectral, 400 to 700nm |
| Incubation | Cell culture and 24-well plate format |
| Advantages and techniques |
High-resolution and extended resolution (AiryScan)
confocal
Spectral unmixing Live cell confocal imaging - FRET, FRAP |
| Software |
Zeiss Zen software (free download)
FIJI ImageJ (free download - 2D analysis) Imaris by Bitplane (licensed) Huygens deconvolution (licensed) |
| Software |
Huygens Professional version 20.04
Modules include:
|
| Description | Deconvolution is an image restoration technique that uses mathematical algorithms to remove blur from an image, while at the same time reducing other degrading effects such as noise and aberrations. Huygens uses state-of-the-art algorithms including the Classic Maximum Likelihood Estimation (CLME) and Good's Roughness Maximum Likelihood Estimation (GLME). Widefield, confocal, multi-photon, and STED images can all be deconvolved. A z-stack with optimized X-Y-Z sampling is required for deconvolution. |
Standard immunofluorescence microscopy (both widefield and confocal) allows one to image 4 colours sequentially without significant spectral overlap. High-multiplex imaging systems go beyond this practical limit to visualize 6, 8, or even up to 40 labeled antibodies in a single tissue slice. The Hyperion Imaging Mass Cytometer (IMC) replaces fluorescent tags with up to 40 metal isotopes: a pulsed laser in the IMC ablates 1um-diameter spots across the tissue which are then analyzed with time-of-flight (TOF) mass spectrometry. Imaging Mass Cytometry is therefore an extremely powerful technique for studying complex cellular processes and interactions at the intact tissue level.
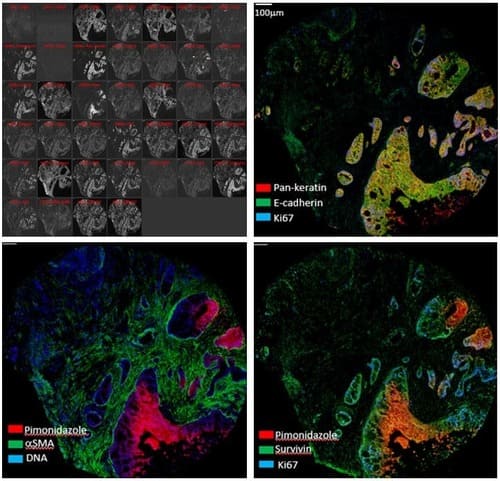
| Model |
Fluidigm Hyperion Imaging System, comprising:
|
| Channels | 135 |
| Frequency | 200 pixels / s |
| Detection limit | ≥ 400 copies per um2 |
| Wet tissue thickness for full ablation | ≤ 7 um |
| Addressable sample size on slide | ≥ 15mm x 45mm |
| Optical view of sample | ≥ 250um x 250um |
| File type | .mcd, OME-TIFF, Multipage TIFF, .txt |
| Scan area | ≥ 1mm2 / 2hr (@ 200 Hz) |
| Advantages and techniques | High-multiplex imaging of tissues sections |
| Software |
MCD viewer (free download) FIJI ImageJ (free download) Cell Profiler (free download) Halo (Indica Labs) |
In addition to confocal and widefield fluorescence microscopes, the AOMF is home to several specialty instruments. These include a Raman microscope, laser microdissection, and an automated Fluorescence In Situ Hybridization (FISH) system at our PMCRT site. Our TGH site has a Total Internal Reflection Fluorescence (TIRF) microscope for particularly thin optical sections adjacent to the coverslip, as well as a full cell culture suite. And our Krembil site sports a stereology system for rigorous cell counting (primarily for neuroscience).
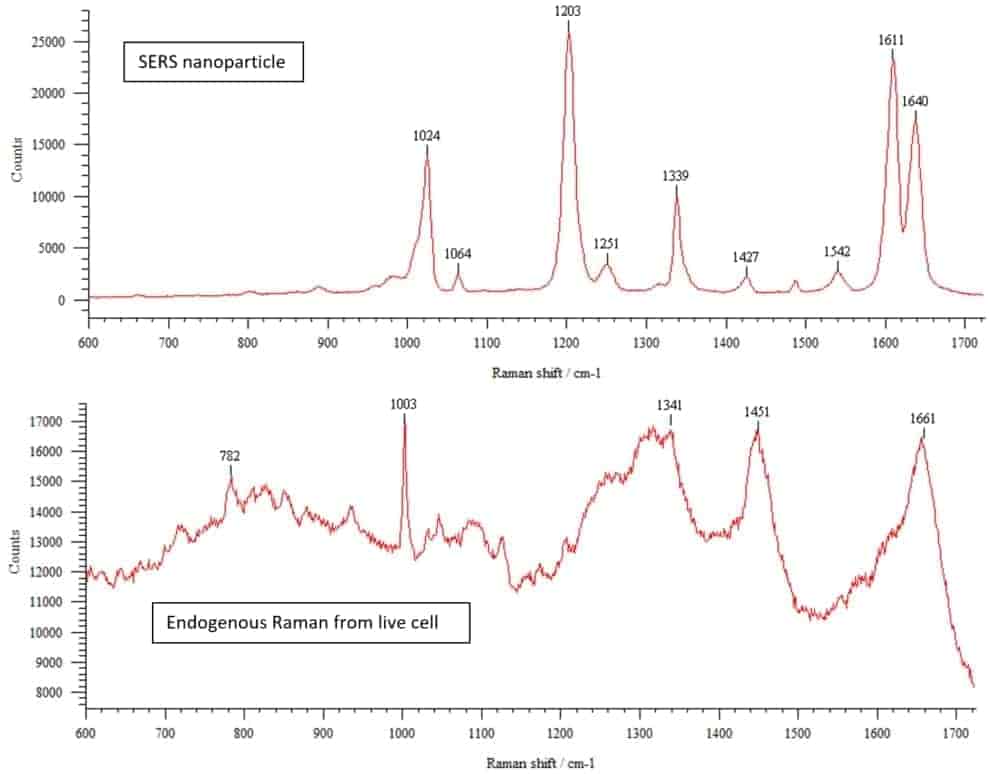
| Model |
Renishaw inVia Confocal Raman Micro-spectrophotometer
Leica DMI 6000 inverted microscope stand |
| Light Sources |
Lasers for Raman:
|
| Objective lenses |
N Plan 2.5x / 0.05 NA (not suitable for Raman)
HCX PL FLUOTAR 5x / 0.15 NA HC PLAN APO 20x / 0.70 NA N Plan 20x / 0.45 NA (long working distance) HCX PL APO 40x / 0.85 NA CORR HCX PL APO 63x / 1.20 NA water immersion CORR HCX APO 100x / 1.3 NA oil immersion (poor IR transmission) |
| Filter sets |
|
| Cameras |
RenCam CCD for spectroscopy (1024 x 256, deep depletion)
Lumenera 3-1M cooled monochrome CCD for fluorescence |
| Advantages and techniques |
Raman spectroscopy, both spatially resolved and in
solutions
3 Excitation wavelengths Raman mapping (low-res imaging) Fluorescence/transmitted/reflected light contrasts for finding the specimen |
| Software | Renishaw Wire 3.0 |
| Model |
MMI CellCut laser capture microdissection
Olympus IX81 inverted microscope stand |
| Light Source |
X-Cite 120 metal halide lamp (fluorescence)
100W halogen lamp (brightfield) |
| Objective lenses |
UPlanFL N 4x / 0.13 NA
UPlanFL N 10x / 0.3 NA Ph1 LUCPlanFL N 20x / 0.45 NA Ph2 LUCPlanFL N 40x / 0.60 NA Ph2 LUCPlanFL N 60x / 0.70 NA |
| Filter sets |
|
| Camera | MMI CellCamera CXA285cF colour CCD |
| Advantages and techniques |
Laser Capture Microdissection of fixed slides for DNA/RNA
sequencing.
Special membrane slides and collection caps are required. |
| Software | MMI CellCut software |
| Model |
Leica SR GSD 3D
Leica DMi8 inverted Stand |
| Light sources |
Sola Light Engine LED for regular live cell imaging (DAPI,
FITC, CY3, CY5 filters)
405 nm depletion laser for STORM/GSD super-resolution 488 nm laser (STORM/GSD/TIRF) 560nm laser (STORM/GSD/TIRF) 642nm laser (STORM/GSD/TIRF) |
| Objective lenses |
HC PL Apo 10x/0.45 NA
N PL 20x/0.40 NA PH1 HC PL Apo 20x/0.8 NA HC PL Apo 40x/0.85 NA HC PL Apo 100x/1.47 NA oil immersion, TIRF HC PL Apo 160x/1.43 NA, oil immersion, GSD |
| Cameras |
Andor iXon Ultra 897 EMCCD
PCO Edge sCMOS |
| Incubation | Tokai Hit stage-top incubator |
| Advantages and techniques |
STORM / GSD super-resolution:
Live cell imaging |
| Software |
Leica LAS-X software
FIJI ImageJ (free download - 2D analysis) |
| Description | Our PMCRT site has a fully equipped tissue culture lab, with a biosafety cabinet, incubators, cryogenic storage, centrifuge, 4 deg C fridge and -20 deg C freezer for sample storage. Users from off-site may request access to this equipment plus assistance from AOMF staff. Users generally provide their own consumables. |
| Model | Leica DMRB Stand |
| Light Sources |
X-Cite 120 metal halide lamp (fluorescence)
100W halogen lamp (brightfield) |
| Objective lenses |
HC Plan Fluotar 5x/0.15 NA
HC Plan Fluotar 10x/0.30 NA Plan Fluotar 20x/0.50 NA Plan Fluotar 40x/1.0 NA oil immersion Plan Fluotar 63x/1.3 NA oil immersion |
| Filter sets |
|
| Camera | Retiga 2000R Cooled Colour CCD |
| Advantages and techniques | Stereological imaging and cell counting |
| Software |
MBF Stereo Investigator
FIJI ImageJ (free download - 2D analysis) |
Each site has at least one image analysis station with FIJI ImageJ for 2D analysis, Imaris (by Bitplane) for 3D rendering and quantification, and HALO (by Indica Labs) for whole-slide analysis of scanned tissue sections. Huygens Deconvolution software is available on analysis stations as well as directly on some of the confocal microscopes (one concurrent license). Instrument-specific software is also available, such as off-line versions of Nikon Elements, Zeiss Zen, Leica LAS-X, Xenogen Living Image, and more.
| Description |
This analysis station is dedicated to one of our 5 Halo
licenses for whole-slide analysis of scanned brightfield
or fluorescence tissue sections. Most slide formats are
supported including:
|
| Hardware |
HP Z840 desktop computer
Intel Xeon CPU E5-2623 v3 (3.00 GHz, 4 Cores, 8 Threads) 32GB RAM Nvidia GeForce RTX 4080 SUPER GPU (10240 CUDA cores, 16GB) HP Z30i 30” LED monitor (2560 x 1600) |
| Software |
AI Nuclear Segmentation
Aperio Imagescope version 12.4 (Leica) FIJI ImageJ Version 1.54 |
| Description | This analysis station hosts one of our 5 Halo licenses for whole-slide analysis of scanned brightfield or fluorescence tissue sections. It can also be used for ImageJ (2D analysis), Imaris (3D rendering and analysis) and Living Image (in vivo imaging analysis). |
| Hardware |
HP Z640 desktop computer
Intel Xeon CPU E5-2650 v4 (2.20 GHz, 12 Cores, 24 Threads) 32GB DDR4-2400 RAM Nvidia Quadro M2000 GPU (768 CUDA cores, 4GB) HP Z32x 31.5” LED monitor (3840 x 2160) |
| Software |
Halo version 3.6 (Indica Labs)
Aperio Imagescope version 12.4 (Leica) Imaris version 10.1 (Bitplane) Xenogen Living Image 4.8 FIJI ImageJ Version 1.54 |
| Description | This analysis station hosts one of our 5 Halo licenses for whole-slide analysis of scanned brightfield or fluorescence tissue sections. It can also be used for ImageJ (2D analysis), Huygens Professional (Deconvolution), and Imaris (3D rendering and analysis). |
| Hardware |
Dell Precision 7920 Computer
Intel Xeon Silver 4214R CPU (2.40GHz 12 Cores, 24 Threads) 32GB RAM NVIDIA Quadro RTX 5000 GPU (1384 Tensor Cores, 48 NT Cores, 16GB) HP ZR30w 30 inch LCD Monitor (2560 x 1600) |
| Software |
Halo version 4.2.6 (Indica Labs) - with AI Nuclear
Segmentation
Aperio Imagescope version 12.4.3 (Leica) Imaris version 10.1.1 (Bitplane) Huygens Professional version 25.10 deconvolution FIJI ImageJ Version 1.54 |
| Description | This analysis station hosts one of our 5 Halo licenses for whole-slide analysis of scanned brightfield or fluorescence tissue sections. It can also be used for ImageJ (2D analysis) and Volocity (3D rendering and analysis). |
| Hardware |
Dell Precision 7820 desktop computer
Dual Intel Xeon Silver 4112 (each CPU 2.6GHz, 4 Cores, 8 Threads) 64GB DDR4 RAM (2666MHz) Nvidia Quadro RTX5000 GPU (3072 CUDA cores, 16GB) |
| Software |
Halo version 3.0311 (Indica Labs)
Aperio Imagescope version 12.3.2 (Leica) Volocity FIJI ImageJ (2D analysis) |
| Description | This analysis station hosts one of our 5 Halo licenses for whole-slide analysis of scanned brightfield or fluorescence tissue sections. It can also be used for ImageJ (2D analysis), Huygens Professional (Deconvolution), and Imaris (3D rendering and analysis). |
| Hardware |
Dell Precision 7810 desktop computer
Dual Intel Xeon CPU E5-2623 v3 (each CPU 3.0GHz, 4 Cores, 8 Threads) 128GB DDR4-2400 RAM Nvidia Quadro M2000 GPU (768 CUDA cores, 4GB) |
| Software |
Halo version 3.0311 (Indica Labs)
Imaris version 9.5.1 (Bitplane) Hugyens Professional deconvolution FIJI ImageJ (2D analysis) |
| Description |
Halo is a powerful analysis platform for whole-slide
analysis of scanned brightfield or fluorescence tissue
sections. Most slide formats are supported including:
|
| Software | Halo version 3.0311 by Indica Labs |
| Description |
Imaris is a powerful platform for visualization and
analysis of 3D datasets, particularly confocal z-stacks
and lightsheet volumes. Our 3 licenses include most of the
available analysis modules including:
|
| Software | Imaris version 9.6 by Bitplane (now Oxford Instruments) |
| Description | Deconvolution is an image restoration technique that uses mathematical algorithms to remove blur from an image, while at the same time reducing other degrading effects such as noise and aberrations. Huygens uses state-of-the-art algorithms including the Classic Maximum Likelihood Estimation (CLME) and Good's Roughness Maximum Likelihood Estimation (GLME). Widefield, confocal, multi-photon, and STED images can all be deconvolved. A z-stack with optimized X-Y-Z sampling is required for deconvolution. |
| Software |
Huygens Professional version 20.04 Modules include:
|
free download
free download
free download
free download
(Click for full fee schedule)
| Service | Type of Instrument | UHN rate | External Academic rate * |
|---|---|---|---|
| Training / Full Service | Confocal / Multiphoton / Super-resolution | $105/hr | $130/hr |
|
Regular usage
(unassisted) |
Confocal / Multiphoton / Super-resolution | $32 to $36/hr | $38 to $43/hr |
| Full Service | Whole-slide scanning: 20x brightfield (BF) | $9/slide | $12/slide |
| Full Service | Whole-slide scanning: 20x fluorescence (FL) | $15/slide | $18/slide |
| Training / Full Service | Widefield | $85/hr | $105/hr |
|
Regular usage
(unassisted) |
Widefield | $14 to $16/hr | $16 to $19/hr |
| Training / Full Service | Image analysis | $85/hr | $105/hr |
|
Regular usage
(unassisted) |
Image analysis | $14/hr | $16/hr |
| After Hours discount | All instruments with permission | - 15% | - 15% |
* Corporate rate is 2x External Academic rate
To protect the instruments and ensure optimum utilization, users must be trained by AOMF staff on each instrument before access will be granted. Our individual hands-on training sessions are 1 to 4 hours in length depending on the complexity of the instrument and the user's prior experience. There is a maximum of 2 trainees per session. Contact AOMF staff for a free consultation to ensure that we set you up on the most appropriate instrument.
This course is an excellent introduction for anyone who plans to get started with our high-end microscopes (confocal, multi-photon, super-resolution, etc.) but does not yet have fluorescence microscopy experience. You start by performing basic fluorescence microscopy and capturing an optimized multi-channel image. You will learn how to align the microscope for Koehler illumination and perform 4 transmitted-light contrast techniques: Brightfield, “Dirty Brightfield”, DIC and Phase Contrast. Finally, you will explore the 4 main elements of a good fluorescence microscope: lamps, filter sets, objective lenses, and cameras. Contact AOMF staff to book this one-on-one training session (groups of 2 are also permitted).
Prerequisite: Please watch this 40 min video before arriving for your course.
This week-long comprehensive course takes the student through all aspects of fluorescence microscopy using dynamic presentations (25%) and hands-on training and experience on the AOMF's advanced microscopes (75%). Topics include: fundamentals of microscopy, contrast and resolution, confocal, spinning-disk confocal, two-photon, live-cell imaging, multi-channel timelapse acquisition, advanced confocal techniques (FRAP, FRET, spectral unmixing), super-resolution, image processing and quantitative analysis (calibrations, background corrections, noise removal, co-localization, deconvolution). The course includes 30hrs of microscope instruction, a course notebook with presentation slides and lab exercises, and refreshments and lunch each day.
Date: Due to COVID19, the 15 th annual Comprehensive Course on Fluorescence Microscopy has not yet been announced.
Prerequisites: None, but it is expected that users have some basic biology/biomedical training.
Who should take this course? - Graduate students, technicians, research associates, and postdocs who require optical microscopy (particularly confocal) for their projects. Even if you already have used confocal extensively, this course will fill in the gaps in theoretical knowledge, help you to optimize your imaging, and teach you to get data from your images through careful image processing and analysis. - PI's: We have had a number of PI's take this course over the years, and they now have an excellent understanding of the limits of optical microscopy and what they can reasonably expect from their students and staff.
Course Outline:Cost: The course fee is $850 for UHN, $950 for academic external and $1,900 for industrial customers.
How to Apply: A registration form will be available here upon announcement of the next course.
Upon completion: Graduates of the course receive a non-accredited certificate.
The AOMF invites applicants from around the globe to participate in our International Microscopy Visitors Program (IMVP). The IMVP is a custom, self-paced program combining hands-on training by our expert staff with ample individual practice time on our microscopes for a truly thorough microscopy learning experience.
Suggested Duration: 3 to 10 days
Cost: $250/day per person
Each day will consist of 2hrs of hands-on microscope instruction time followed by 4hrs of unassisted microscope practise time. The program is designed for individuals or groups of no more than 4 participants (discounts for groups may apply). The IMVP can be arranged anytime of the year subject to microscope and staff availability.
The duration of the course is flexible, depending on your microscopy experience and ambitions. A 3-day program would be sufficient to gain a thorough working knowledge of confocal fluorescence microscopy, including:Please contact AOMF if you're interested and we'd be happy to discuss the program with you!
Good resources for learning about confocal fluorescence microscopy and related techniques:
It is helpful to have some background knowledge before jumping onto our sophisticated instruments:
Resources for helping me prepare my samples:
Trying to determine which dishes, fluorophores, antibodies, or fluorescent proteins to use? You may find these links useful. Nevertheless, you're welcome to discuss your planned sample prep with AOMF staff before purchasing reagents, dishes, etc. to make sure they are suitable for our microscopes. For your convenience, the AOMF stocks some coverslips and dishes in various sizes.
Optical Microscopy Users Group (O-MUG):
The Optical Microscopy Users Group discusses topics ranging from new microscopy systems to acquisition to analysis techniques. Each meeting comprises a 30-45min presentation on a selected topic followed by discussion. O-MUG presentations are suitable for all levels of microscope users.
Recordings are available in the O-MUG archives. ▸ Go To Archives
If you are based at one of our 4 sites, feel free to email a staff member at that site directly. Otherwise please contact aomf@uhn.ca and we'll direct your inquiry appropriately.
The AOMF's largest site is in the MaRS Center in the Princess Margaret Cancer Research Tower (PMCRT). This site is equipped for slide scanning, confocal, multiphoton, widefield, super-resolution, and more.
Address:
101 College St., Room 15-305
Toronto, ON, CANADA, M5G 1L7
Staff:
Judy Cathcart (judith.cathcart@uhn.ca)
Wesley Walker (wesley.walker@uhn.ca)
Courtney McIntosh (courtney.mcintosh@uhn.ca)
Tag (Xiaotian) Yu (tag.yu@uhn.ca)
James Jonkman (james.jonkman@uhn.ca)
Henrietta "Henny" Bennett (henrietta.bennett@uhn.ca)
The AOMF's TGH site in the Max Bell Research Center (MBRC) is equipped for confocal, widefield, TIRF, and image analysis with an emphasis on live-cell imaging.
Address:
101 College St., MBRC Room 4R410
Toronto, ON, CANADA, M5G 1L7
Staff:
Feng Xu (feng.xu@uhn.ca)
Wesley Walker (wesley.walker@uhn.ca)
The AOMF's Krembil Research Institute site (at Toronto Western Hospital) is named after generous benefactors Bob and Joan Wright. The WCIF is equipped for confocal, multi-photon, widefield, stereology and image analysis with an emphasis on neuroscience and vision applications.
Address:
60 Leonard Avenue, Room 7KD-492
Toronto, ON, M5T 0S8
Staff:
Tag (Xiaotian) Yu (tag.yu@uhn.ca)
Wesley Walker (wesley.walker@uhn.ca)
Krembil personnel: Take the KDT service elevators to 7th floor - the WCIF is opposite the elevators.
External visitors: Please make your way to the Second Cup on the ground floor at Toronto Western Hospital, then call Tag or Wesley and they will meet you to escort you to the facility.
The AOMF's PM site is equipped for confocal, widefield, and image analysis.
Address:
610 University Ave., Room 7-305
Toronto, ON, CANADA, M5G 2M9
Staff:
James Jonkman (james.jonkman@uhn.ca)
Wesley Walker (wesley.walker@uhn.ca)
Our experienced staff can help you to choose the best instrument/technique for your application. We recommend that you contact us for a free consultation to discuss your project. When you're ready to start:
Review our Fee Schedule.
Review our Policies regarding bookings, safety, and temporary COVID safety measures. This document is quite long, but we really do want you to read it before you start!
Please follow the Getting Started Instructions for creating your new Stratocore account and accessing the AOMF calendars.
The AOMF welcomes all users including corporate users, external academics, and of course internal (UHN) users. A variable fee schedule applies.
Please take a look at the resources section.
Please take a look at the resources section.
The Resolution Benchmarking Platform is a community resource for microscopists to measure and compare the resolution of their microscopes with similar measurements from other instruments around the world. A benchmarking protocol is provided in the user guide. Access to the platform is free and open to any member of the global imaging community. Registration is required to submit and view images.
* For a clearer view, click the arrow icon in the bottom-right corner to open the chart in full-screen mode. Once opened, you can use the zoom-in button to enlarge the details.